Alkanes can be used in free-radical substitution reactions to produce halogenoalkanes. Give equations for the propagation steps in the reaction of butane to form 2-chlorobutane.
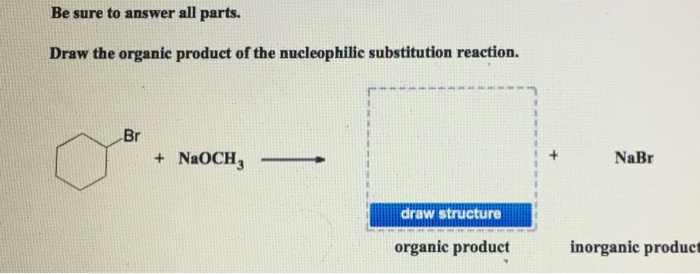
Solved Be Sure To Answer All Parts Draw The Organie Product Chegg Com
The general mechanisms for each of these reactions are shown below.

. Mechanisms of nucleophilic substitution. Chlorofluorocarbons CFCs are a group of halogenoalkanes currently banned in many. By itself Infrared IR spectroscopy isnt a great technique for solving the structure of an unknown moleculeHowever weve seen that IR spectroscopy can a great technique for identifying certain functional groups in an unknown molecule especially functional groups containing OH or CO.
For instance in an earlier post. 9 t-butyl chloride undergoes solvolysis in 70 water30 acetone at a rate slower than in 80 water20 acetone. This paper is a mechanistic investigation.
2 marks 3 03 Turn over IBMJun1874052. The bromine atom leaves with its bonding electrons as a bromide ion. Ethers are known to be unreactive towards most reagents which makes them excellent reaction solvents.
61 General Nucleophilic Substitution Reactions A deprotonation step is required to complete the reaction when the nucelophile was a neutral atom that bore a proton Example showing deprotonation 4. Give the structure of the substitution product. It is there because only the alpha hydrogen can be removed to form the enolate leaving any other more remote carbons of the aldehyde as a branch.
Do not write outside the box. Diaryl ethers are not cleaved by acids. In the first step we have the nucleophilic attack of the Grignard making the C-C bond and shifting the electrons of the π bond to the oxygen.
The most common reaction of ethers is cleavage of the CO. This is a study of an unusual but interesting reaction developed in the labs of the late Prof P. Q The branch incidentally always occurs on the enolate side of the aldol product.
62 Carbocation Stability Order of Carbocation Stability. 3 2 1 Methyl 63 actorsF A ecting the Rates of S N1 and S N2 Reactions. Remember these are just general mechanisms - for the exam you will have to draw them specific to the reaction in your exam question.
Q You should be able to 1 Write the detailed mechanism for the aldol addition reaction of any. Consider the product of the aldol reaction of propanal. The difference with aldehydes and ketones is that the product of this addition reaction to the carbonyl contains an alkoxy group on the tetrahedral intermediate.
The final product is a nitrile. Br CH2CH3 CH3OH D. Nucleophilic substitution with cyanide ions.
The cyanide ion attacks at the partially positive carbon of the dipole making a high energy transition state. IR Spectroscopy Practice Problems. The reaction of epoxides with TMSCN an organic-soluble and easier-to-handle substitute to traditional cyanide reagents such as KCN and catalytic ZnI 2 gives 12-isocyano alcohols.
As phenols do not undergo nucleophilic substitution reactions even if an excess of HX is used the products from the cleavage of an aryl alkyl ether are a phenol and an alkyl halide. This group is a relatively good leaving. 10 Provide the major organic product of the reaction below and a detailed stepwise mechanism which accounts for its formation.
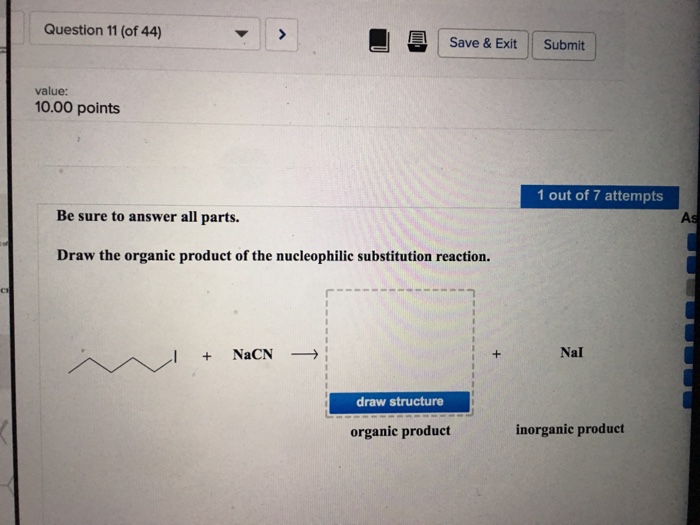
Solved Draw The Organic Product Of The Nucleophilic Chegg Com
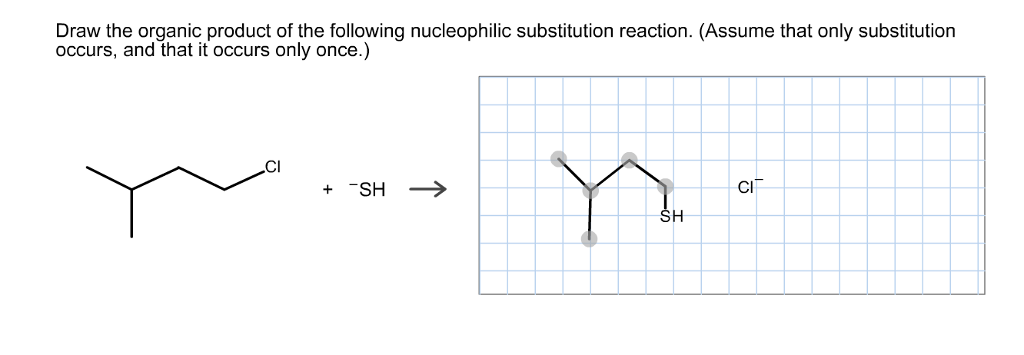
Solved Draw The Organic Product Of The Following Chegg Com
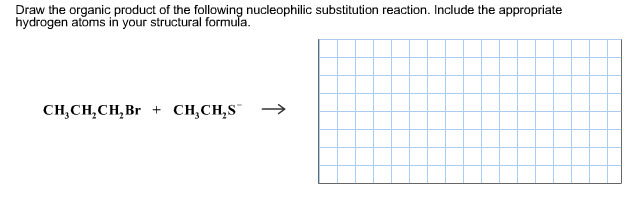
Solved Draw The Organic Product Of The Following Chegg Com
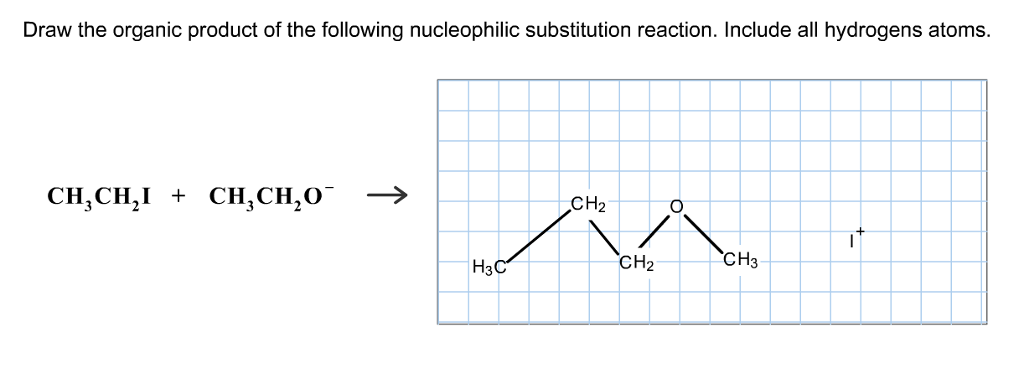
Solved Draw The Organic Product Of The Following Chegg Com
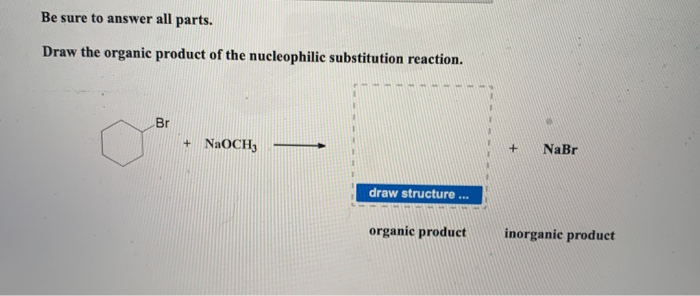
Solved Be Sure To Answer All Parts Draw The Organic Product Chegg Com
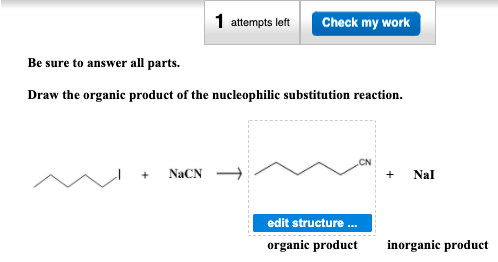
Solved 1 Attempts Left Check My Work Be Sure To Answer All Chegg Com
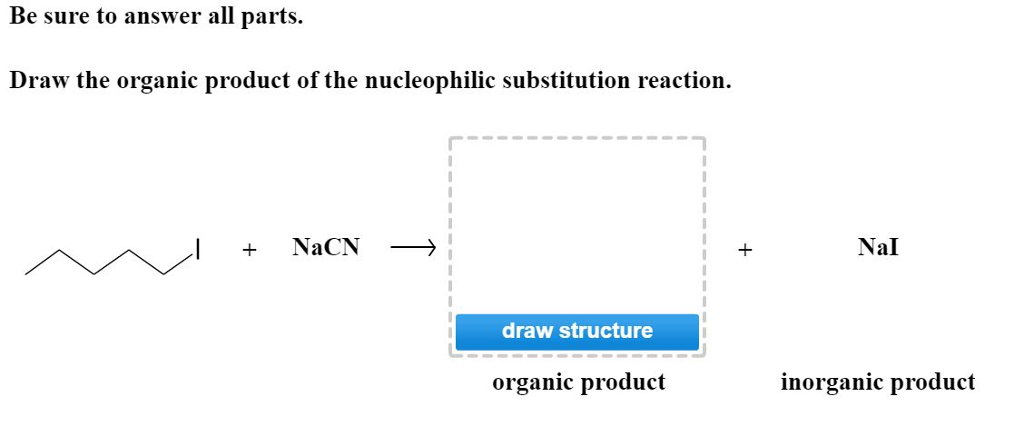
Solved Draw The Organic Product Of The Nucleophilic Chegg Com

Draw The Organic Product Of The Nucleophilic Substitution Reaction Study Com
0 comments
Post a Comment